
Implanted Telemetry in Preclinical Research: Enhancing data quality and animal welfare
Implanted telemetry is a biomedical technology used in preclinical research to monitor an animal’s vital signs such as heart activity (rate and ECG), blood pressure, body temperature, EEG/EMG and activity, transmit biological data in real time to external receivers, allowing scientists to study how the body responds to new drugs or chemicals under natural, stress-free conditions.
Unlike traditional methods that rely on external wires or frequent animal handling, telemetry implantation allows researchers to observe animals while they move freely in their environment. This leads to more reliable, high-resolution data and better reflects real-world drug effects. Telemetry plays a key role in drug development, where understanding a compound’s physiological impact is critical.
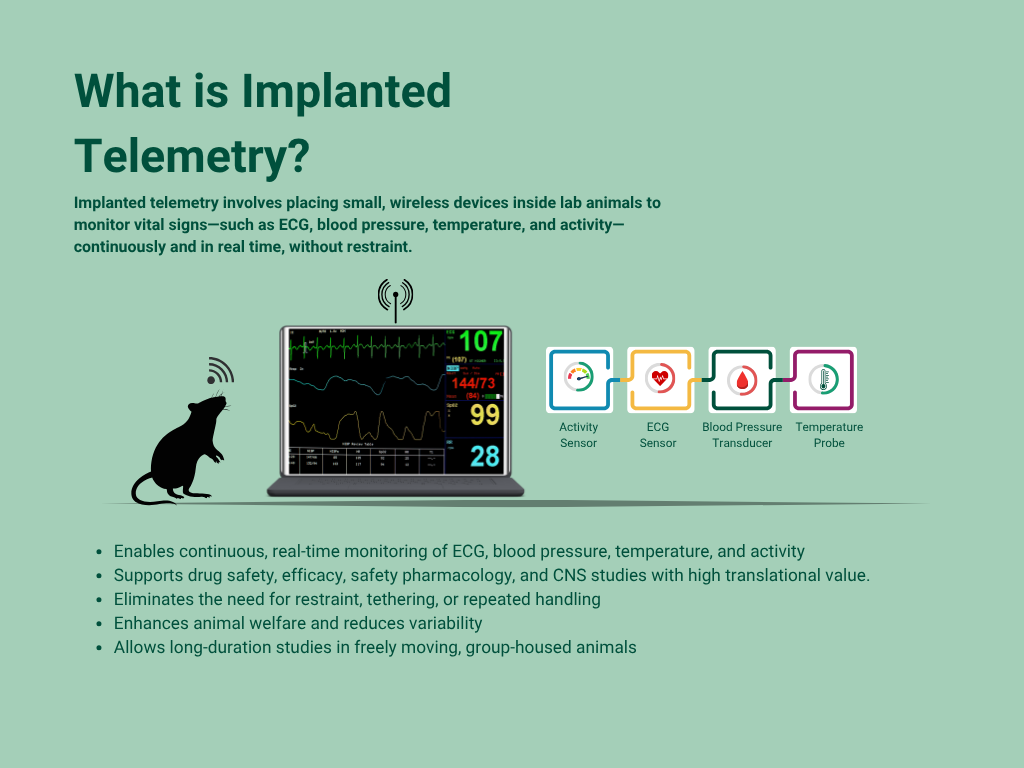
At Biotrial, a leader in GLP-compliant preclinical research, we integrate advanced animal telemetry systems to ensure accurate, ethical, and reproducible data. Our telemetry platforms support critical applications in efficacy and safety pharmacology, along the drug development journey.
Why implanted telemetry is crucial for preclinical research
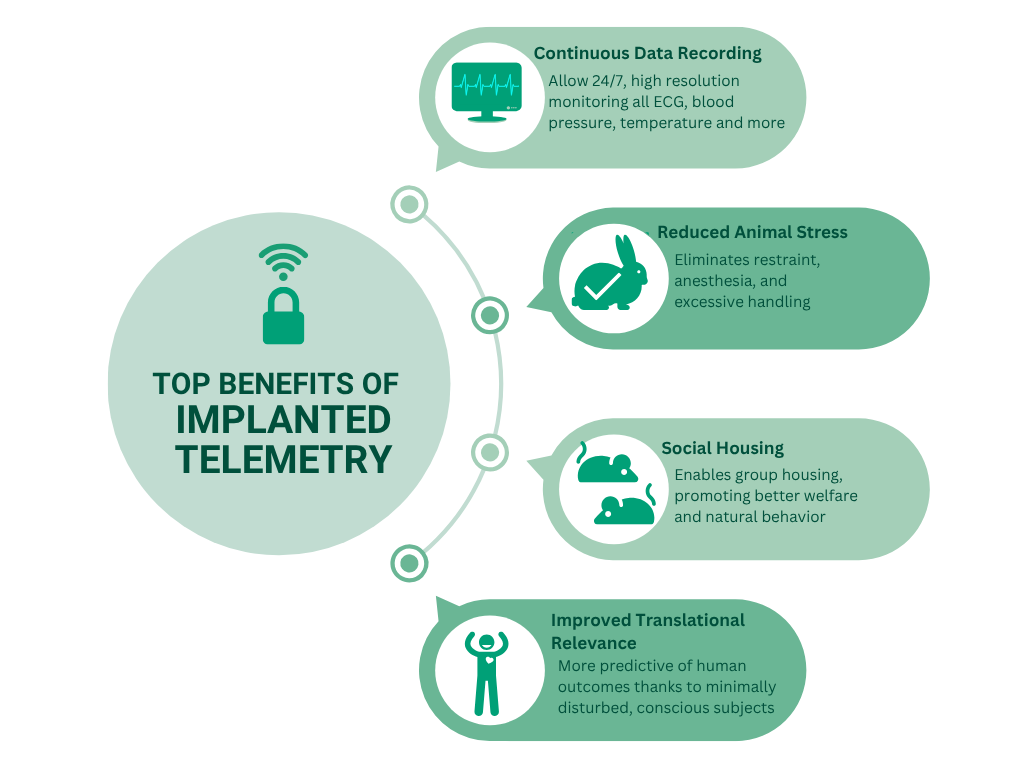
Top benefits of telemetry device implantation in laboratory animals
Continuous Physiological Monitoring: Implanted telemetry enables 24/7 monitoring of vital parameters such as ECG, blood pressure, temperature, and activity, especially during key pharmacokinetic and pharmacodynamic time windows.
- Enhanced Animal Welfare in Preclinical Research: By eliminating repeated anesthesia, handling, and restraint, telemetry device implantation significantly reduces stress-induced variability in animal models.
- Group Housing with Implanted Telemetry: Advanced animal telemetry systems allow animals to remain socially housed, preserving natural behaviors and improving scientific outcomes.
- Translational Relevance: Data from conscious, freely moving animals with implanted telemetry closely mirrors human physiological responses—boosting the predictive power of preclinical studies.
Biotrial Insight: We elevate data precision and ethical standards by combining surgical expertise, refined housing protocols, and fully integrated preclinical telemetry solutions.
From analog systems to digital telemetry precision from analog systems to digital telemetry precision
A decade of Innovation in animal telemetry systems
The evolution of implanted telemetry systems has transformed the field. Early analog telemetry setups were limited by interference, short range, and single-frequency communication. Today’s digital telemetry platforms offer:
- Multiple transmission channels to reduce signal interference
- Extended range and better signal integrity in open or large enclosures
- Remote configuration to minimize human interaction
- Full compatibility with behavioral and cognitive testing environments
What implanted telemetry measures and why it matters
Key physiological parameters captured by telemetry implantation:
| Parameter | Purpose in Preclinical Studies |
| ECG (Electrocardiogram) | Cardiovascular safety, arrhythmia detection, QTc prolongation |
| Blood Pressure (BP) | Hemodynamic effects, vascular response monitoring |
| Core Body Temperature | Fever detection, thermoregulation analysis |
| Locomotor Activity | Behavioral assessment, sedation, stress response |
| EEG (Electroencephalogram) | CNS function, seizure liability, sleep studies, event-related potentials (ERPs) |
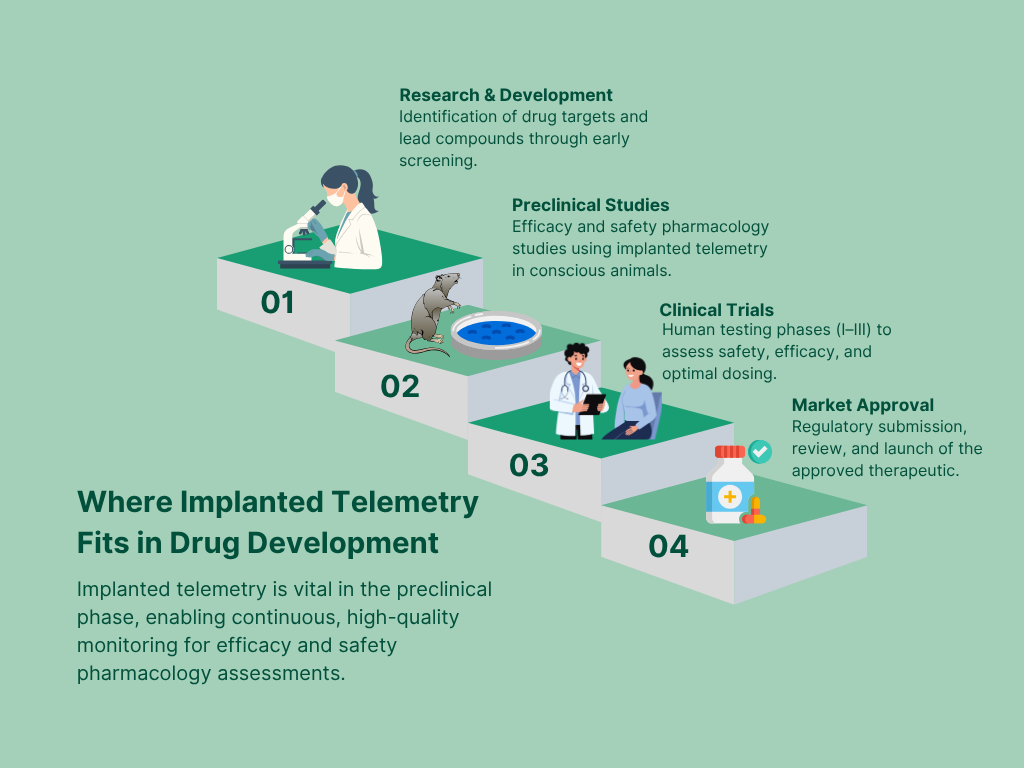
These critical endpoints, continuously recorded through telemetry, offer unparalleled insight into a drug’s efficacy and safety profile—without the need for repeated invasive procedures.
Scientific and ethical advantages of implanted telemetry
One of the most compelling reasons to use implanted telemetry in preclinical research is its ability to refine and reduce animal use, particularly by enabling more efficient study designs and reducing the number of animals needed through continuous, high-quality data collection .
Ethical and scientific benefits:
- Higher statistical power with fewer animals
- Lower variability due to reduced handling and stress
- Strong alignment with the 3Rs: Replacement, Reduction, and Refinement
- Greater reproducibility and sensitivity in safety pharmacology studies
At Biotrial, our animal telemetry systems reflect a commitment to ethical science and robust data integrity.
GLP compliance and study design for telemetry studies
Successful telemetry studies in preclinical research depend on strict compliance with international guidelines and thoughtful experimental design.
Compliance and design tools:
- ICH S7A/S7B Guidance: Define regulatory expectations for safety pharmacology studies[ED1]
- ARRIVE Guidelines: Ensure complete, transparent reporting of animal data
- SYRCLE’s RoB Tool: Reduce bias in design and outcome analysis
Current challenges and future developments in Telemetry
While implanted telemetry systems offer many advantages, a few limitations still exist.
Challenges:
- Post-surgical recovery requires temporary single housing, which must be carefully managed to minimize stress in social species
- Trade-offs between device size and battery life
- Requires specialized surgical teams and post-op care
What the Future holds:
- Development of smaller, longer-lasting implants
- Multi-sensor devices capturing ECG, BP, EEG, and temperature (and more) simultaneously
- AI-driven telemetry data analysis with real-time alerts
Biotrial stays ahead by adopting and testing next-gen preclinical telemetry technologies to maintain innovation leadership.
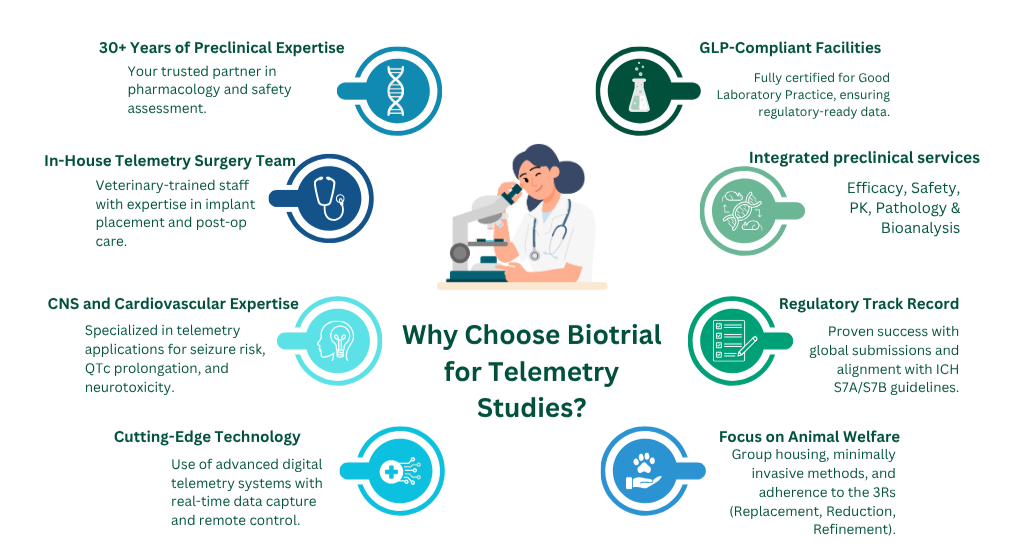
Why Biotrial is the trusted partner for telemetry implantation studies
Partnering with Biotrial means:
- 30+ years of preclinical and telemetry research experience
- Dedicated in-house telemetry acquisition labs and surgery teams
- Full integration across pharmacodynamics, pharmacokinetics and bioanalysis
- GLP- and AAALAC-certified infrastructure and global regulatory success
Whether you’re studying cardiovascular or CNS drug effects, Biotrial’s telemetry services ensure your study is accurate, compliant, and future-ready.
Conclusion: Implanted telemetry is revolutionizing preclinical research
In summary, implanted telemetry is reshaping how scientists approach drug efficacy and safety evaluation in preclinical research. This technology delivers more data with fewer animals—boosting both scientific rigor and ethical responsibility.
At Biotrial, we are committed to delivering top-tier telemetry-enabled preclinical studies. Our expertise in telemetry implantation, data interpretation, and regulatory compliance ensures that your programs advance with confidence and clarity.

