The Use of EEG in Epilepsy Research: An Objective Biomarker Driving Clinical Innovation
Electroencephalography (EEG) is a core tool in epilepsy clinical trials, offering an objective biomarker for seizure detection, enabling drug development teams to monitor disease progression and evaluate treatment effects. As a non-invasive method that records the brain’s electrical activity, EEG provides critical insights into epileptic phenomena—enabling researchers to design better studies, support regulatory interactions and ultimately improve patient care.
At Biotrial, we deliver comprehensive EEG services from equipment provision to centralized expert review and advanced exploration analyses, empowering sponsors to generate high-quality data across all trial phases.
Understanding EEG in Epilepsy Research
What is EEG and Why is it Critical?
Electroencephalography (EEG) records the brain’s electrical signals via electrodes placed on the scalp. This non-invasive method allows detection of abnormal electrical discharges that characterize epileptic seizures and other neurological disorders. In some cases, subcutaneous electrodes can be used for ultra-long monitoring over several weeks, providing valuable insights for complex or hard-to-capture seizure patterns.
EEG serves as an objective biomarker by:
- Capturing real-time seizure activity and interictal epileptiform discharges.
- Quantifying seizure frequency and severity for clinical trial endpoints.
- Monitoring treatment effects over time to assess efficacy and safety.
- EEG’s non-invasive nature and high temporal resolution make it a fundamental measurement system in both early and late-phase clinical studies.

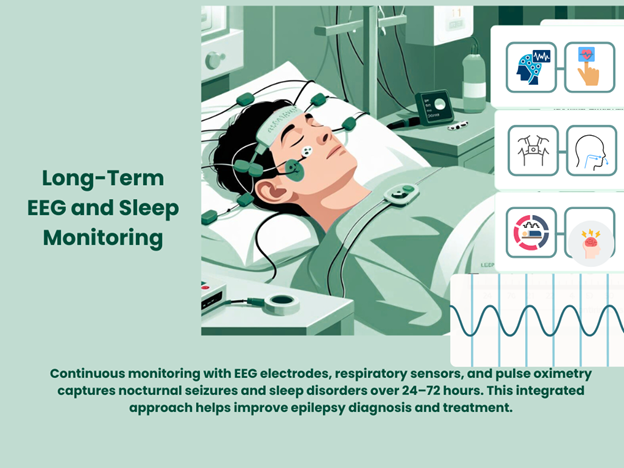
The Importance of Long-Term EEG Monitoring
Capturing Rare and Nocturnal Seizures
Many patients experience seizures sporadically or during sleep, making short EEG recordings insufficient. Biotrial offers long-term EEG monitoring from 24 hours to multiple days, enabling:
- Detection of infrequent and subtle seizure events.
- Identification of nocturnal seizures critical for comprehensive epilepsy management.
- Tracking seizure clusters and cyclical patterns.
- This approach ensures the total seizure burden is accurately measured, which is vital for both patient care and regulatory approval of new treatments.
Complementary Sleep Studies and Apnea Monitoring
Because obstructive sleep apnea (OSA) often coexists with epilepsy and can exacerbate seizures, Biotrial integrates EEG with polysomnography (PSG) to monitor sleep stages, respiratory events, and oxygen saturation. This provides critical context for seizure interpretation and treatment impact.
Ensuring Data Quality and Consistency Across Sites
All EEG data collected in Biotrial epilepsy trials undergoes central review by expert neurologists and epileptologists trained to interpret complex electrophysiological patterns consistently. This process:
- Reduces inter-rater variability and interpretation bias.
- Provides rapid and reliable feedback to support informed decision-making.
- Supports regulatory compliance through documented, reproducible review workflows.
- Central reading also facilitates multi-site trials by standardizing quality and interpretation, regardless of geographical location.
Advanced Exploratory EEG: Quantitative EEG (qEEG)
Moving Beyond Visual Review with Data-Driven Metrics
Biotrial supports quantitative EEG (qEEG), which applies advanced computational techniques to transform EEG signals into rich numerical data. Key qEEG methods include:
- Spectral analysis to quantify brainwave frequencies (delta, theta, alpha, beta, gamma).
- Functional connectivity mapping to assess communication between brain regions.
- Complexity and entropy measures that may correlate with cognitive or treatment response markers.
- Correlation with physical and cognitive endpoints to identify biomarkers predictive of patient outcomes.
- qEEG empowers sponsors with novel endpoints and deeper insights into treatment mechanisms and efficacy, accelerating drug development.
Integration with Sleep and Respiratory Monitoring
Addressing Comorbidities for Comprehensive Patient Care
Epilepsy rarely exists in isolation. Sleep disorders, particularly OSA, significantly affect seizure control and patient quality of life. Biotrial can integrate EEG with PSG and respiratory monitoring to provide:
- Comprehensive assessment of sleep architecture disruptions.
Detection and quantification of apnea events and investigation of their correlation with seizure frequency - Evaluation of adjunctive treatments like CPAP therapy and their effects on epilepsy.
This multi-modal approach ensures trials consider all physiological parameters influencing treatment outcomes.
Biotrial’s Comprehensive EEG Clinical Trial Services
End-to-End Support from Phase I to III
Our services cover the full clinical trial lifecycle, including:
Provisioning and installation of advanced EEG and PSG equipment at clinical sites, or alternatively, setting up a scalable central review data flow using EEG recordings captured with site equipment. We can also work with local equipment, following a specific process to verify the quality and compliance of data recorded at each site. Data consistency can be controlled across centers, which is particularly important in epilepsy studies, where sites often have extensive EEG experience and may be reluctant to adopt alternative devices
- Training and ongoing support for site personnel and laboratory technicians.
- Central quality control and feedback to sites.
- Centralized data review, archiving, and regulatory-compliant documentation.
- Exploratory qEEG analytics and integration with other physiological measurement systems (blood pressure, ECG, etc.).
- Flexible solutions with built-in scalability for small pilot studies or large multicenter trials worldwide

Why Partner with Biotrial for Your Epilepsy Trials?
Expertise, Quality and Global Reach
Biotrial brings decades of neuroscience and epilepsy clinical research experience to your study. We provide:
- Deep scientific expertise and a multidisciplinary team.
- State-of-the-art EEG, PSG, and ambulatory monitoring technologies.
- Comprehensive site support with multilingual, GCP-trained staff.
- Innovative analytical capabilities including qEEG and multi-parameter data fusion .
- Commitment to quality data ensuring regulatory confidence.
- Customized trial design support aligned with your scientific and clinical goals.
Conclusion: Advancing Epilepsy Research with Robust EEG Solutions
Electroencephalography (EEG) stands as an indispensable, objective biomarker in epilepsy clinical trials—providing rich, high-quality data essential for understanding seizure dynamics, evaluating novel treatments, and improving patient outcomes. Through long-term monitoring, centralized expert review, and advanced exploratory techniques like quantitative EEG (qEEG), researchers gain unparalleled insight into the complex neurological underpinnings of epilepsy.
At Biotrial, our comprehensive EEG services combine cutting-edge technology, scientific expertise, and global site support to deliver reliable, reproducible results that accelerate drug development and regulatory approval. By integrating EEG with complementary modalities such as polysomnography and apnea monitoring, we ensure a holistic approach addressing the multifaceted needs of epilepsy patients.
Choosing Biotrial means partnering with a trusted, innovative CRO committed to advancing epilepsy research through quality, precision, and collaborative care. Together, we can unlock new treatment possibilities and improve lives—one brainwave at a time.

