EEG Biomarker Portfolio
Biotrial’s Core Lab team is comprised of experts who specialize in supporting clinical sites across the globe with EEG measurements. This includes providing devices, training site staff to perform recordings, supporting the sites throughout the trial, and centrally analyzing the data captured.
EEG endpoints that can be centrally managed include:
| Domain | Test/Marker | Measure | |
|---|---|---|---|
| Resting EEG | qEEG Seizure & Spike detection and quantification Qualitative neurologist review | Safety assessment (neurologist) Spectral parameters (rel & abs power) | Brain maps Spectral ratios Source localization Functional connectivity |
| Event-Related Potentials | Cognitive potentials Auditory gating Gamma evoked Specifics upon request | P300 (oddball paradigm), Auditory Steady State Response Mismatch Negativity Memory (LMT), Error Related Negativity (flanker task) | P50, N100 Auditory Gating Sustained Attention to Response (SART) |
| Sleep EEG | PSG Sleep microstructure Spectral analysis Maintenance of Wakefulness Test | Sleep staging (ASSM 2007 or R&K) Apnea, Hypopnea, Arousals, Periodic movements, etc. | Spindle analysis: Count, Density, Position, Frequency, Amplitude, Duration, Power, etc… |
| Preclinical EEG | Sleep Resting EEG ERP | Sleep scoring Spectral parameters Auditory Steady State Response | Seizure count |
Safety EEG
Safety EEG: Monitoring for Treatment Safety and Pre-Existing Abnormalities
Safety EEG is a non-invasive procedure that is used to monitor the electrical activity of the brain. It is used in clinical trials to assess the safety of investigational drugs and to evaluate participants for pre-existing EEG abnormalities. Safety EEG recordings are typically collected during a participant’s screening visit and at regular intervals throughout the trial. The recordings are reviewed by a neurologist to identify any signs of pro-convulsive effects of the investigational drug or pre-existing EEG abnormalities.
For certain participant profiles, such as those with a history of epilepsy or other neurological disorders, more frequent EEG monitoring or qualitative reviews may be required. Qualitative reviews involve a detailed analysis of the EEG recording by a neurologist to identify any subtle changes in brain activity. Each safety EEG recording is quality-controlled by a specialized technician to ensure data quality. If any quality issues are identified, feedback and additional information or training are provided to the study site.
After the quality control step, the EEG recording is subjected to a semi-automated analysis. During this analysis, the EEG is first automatically processed using specialized software. The result of this processing is then reviewed by a neurologist to identify any significant abnormalities.
The results of the safety EEG analysis are reported back to the study site and the sponsor. Expert reports are also provided at the end of the project or at specified times during the trial.
- Standard recording protocol of 20 to 40 minutes, including photic stimulation & hyperpnoea
- A long-duration continuous recording lasting up to 48 hours. This is the gold standard for detecting epileptogenic activity
Examples of recording paradigms:
Quantitative EEG (qEEG)
Biotrial offers a comprehensive range of qEEG services to support our clients in the development of new drugs and treatments for neurological disorders. qEEG is a non-invasive method that is used to measure the electrical activity of the brain. It can be used to assess the impact of a compound on brain function by measuring changes in brain waves in different frequency bands.
Biotrial’s qEEG services help our clients to:
- Establish proof of mechanism: identify the specific brain mechanisms that are affected by a compound. This information can be used to validate the drug target and to guide the development of new and more effective drugs and treatments.
- Define the compound’s EEG signature: create a unique EEG signature for a compound. This signature can be used to track the compound’s effects on brain function over time and to compare it to the effects of other known drugs.
- Compare to the action of existing known drugs: compare the EEG signature of a new compound to the EEG signatures of existing known drugs. This information can be used to understand how the new compound works and to predict its potential therapeutic effects.
- Understand the physiological effects of their compound (drowsiness, cognition, etc.): assess the physiological effects of a compound, such as drowsiness and cognition. This information can be used to design safe and effective clinical trials and to develop new and innovative drugs and treatments.
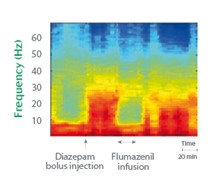
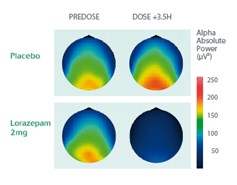
Example: Characterization of the CNS Effects of Benzodiazepines
Time-frequency representation of Diazepam effect on rodents and reversal by Flumazenil.
Cross-over study on 20 healthy volunteers administered with Lorazepam (brain mapping representation).
Event-Related Potentials
The EEG signal can vary when the subject is the object of a sensory, motor, or cognitive stimulus. Event-Related Potentials (ERPs) are a powerful tool for measuring brain activity in response to stimuli. Biotrial provides state-of-the-art ERP services for clinical research, including EEG recording, stimulus delivery, and response recording.
Event-Related Potentials (ERPs) are a type of electroencephalography (EEG) that measures the electrical activity of the brain in response to a stimulus. ERPs are typically measured using a series of electrodes placed on the scalp. When a stimulus is presented, the electrodes measure the electrical changes in the brain that occur in response to the stimulus.
ERPs can be used to study a wide variety of cognitive processes, including attention, memory, perception, and decision-making. They can also be used to study the effects of different drugs and treatments on the brain.
Biotrial provides state-of-the-art ERP services for clinical research. Our services include:
- EEG recording
- Stimulus delivery
- Response recording
We also offer a variety of data analysis services, including ERP analysis, statistical analysis, and machine learning analysis.
.
ERPs are a valuable tool for clinical research because they can provide insights into the brain’s response to different stimuli and treatments. ERPs can be used to develop new treatments for a variety of neurological disorders, such as sleep apnea, Alzheimer’s disease, and Parkinson’s disease.

Polysomnography (PSG)
PSG recordings can be used to investigate a compound’s effect on participants’ sleep. These recordings are performed overnight within a clinical setting and allow to determine a participants hypnogram as well as a full set of sleep parameters such as sleep latency, total sleep time, sleep efficiency, time per stage, WASO, etc…
In addition to EEG we record EOG, EMG, ECG and, for specific sleep apnea investigations it is also required to record breathing.
Clinical Site Support & Equipment
Biotrial’s Core Lab offers state-of-the-art equipment and continuous staff support specifically designed for clinical trials.
- Global shipment of EEG/PSG devices for collection of data
- Site training and 24/7 support for worldwide clinical sites
- Immediate replacement of any defective equipment
- Continuous quality monitoring of Data recordings with feedback to study sites
Biotrial’s fully–validated system ensures high-quality EEG recordings. The study-specific kits are user-friendly and allow for quick set-up at any clinical site (CPU or hospital) involved in a clinical trial. Our equipment supports standard EEG as well as advanced EEG-based assessments, such as PSG or ERP, in addition to 24-hour (or longer) ambulatory recordings.
EEG Analysis & Reports
EEG recordings are processed by our expert signal processing team. The Biotrial Core Lab uses validated signal processing tools and medical expertise to implement study data analyses:
- Qualitative reviews by board-certified neurologists
- qEEG: spectral analysis of the EEG recording, brain maps to provide a graphic representation of results
- ERP (auditory or visual event-related potentials): brain mapping and graphical representations
- Polysomnography (sleep EEG): sleep scoring, spectral analysis, spindle analysis
- Exploratory analysis: study specific algorithm implementation
In order to ease the understanding and interpretation of the EEG signal and results, our team has developed a series of tools used to provide graphic, easy-to-grasp representations of these results. These are reported back to the sponsor team at regular times during a project.
Data Management, Statistical Analysis & Report Writing
All activities are covered by Biotrial SOPs, guaranteeing data quality, process efficiency & compliance.
- Custom-made database set up and sponsor-specific data transfer protocols
- Statisticians & Medical Writers with extensive EEG experience
Consulting & Design Services
- Biotrial Experts can help you select the optimal EEG modality and the relevant assessment method for your project
- Animal EEG data can easily be generated in our preclinical facilities to support sponsor development strategies
- We offer interpretation of data and advice on the overall development plan for your compound.


