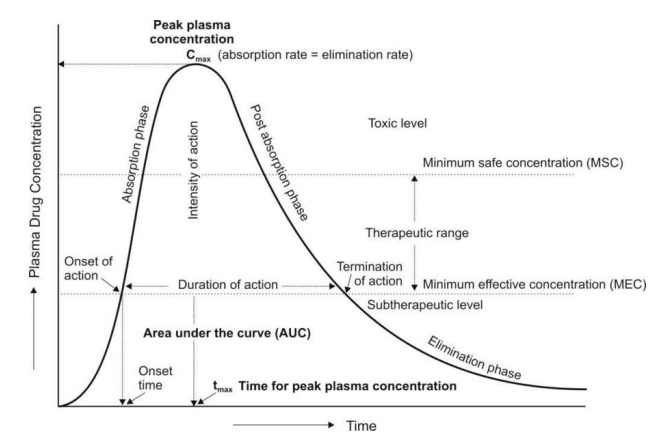
Pharmacokinetics
Our experienced Pharmacokineticists offer non-compartmental and compartmental analyses and PK/PD analyses.
Our experience includes performing final analyses for PK endpoints as well as providing continuous PK reporting throughout a trial to assist sponsors in decision-making, e.g. for dose-escalation decisions.