
CAR-T cell therapy: driving innovation from preclinical research to clinical success
CAR-T therapy emerged from decades of research in immunology and gene engineering, combining advances in T-cell biology, molecular targeting, and cell therapy manufacturing. The first successful clinical trials in the early 2010s demonstrated remarkable remission rates in patients with certain blood cancers, paving the way for FDA-approved CAR-T therapies. These milestones validated the concept of genetically reprogramming immune cells and set the stage for expanding CAR-T approaches to more complex cancers.
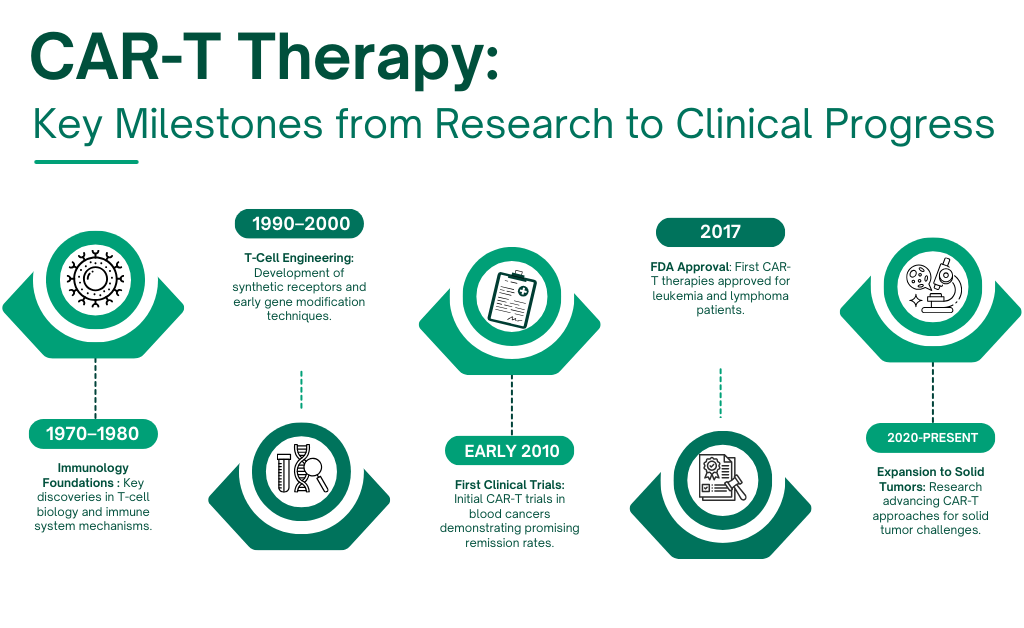
Chimeric Antigen Receptor T-cell (CAR-T) therapy represents a major frontier in oncology, offering a highly specific and personalized approach to cancer treatment. By genetically engineering a patient’s T cells to express synthetic receptors that recognize tumor-associated antigens, CAR-T cells can be redirected to seek out and destroy cancer cells with precision.
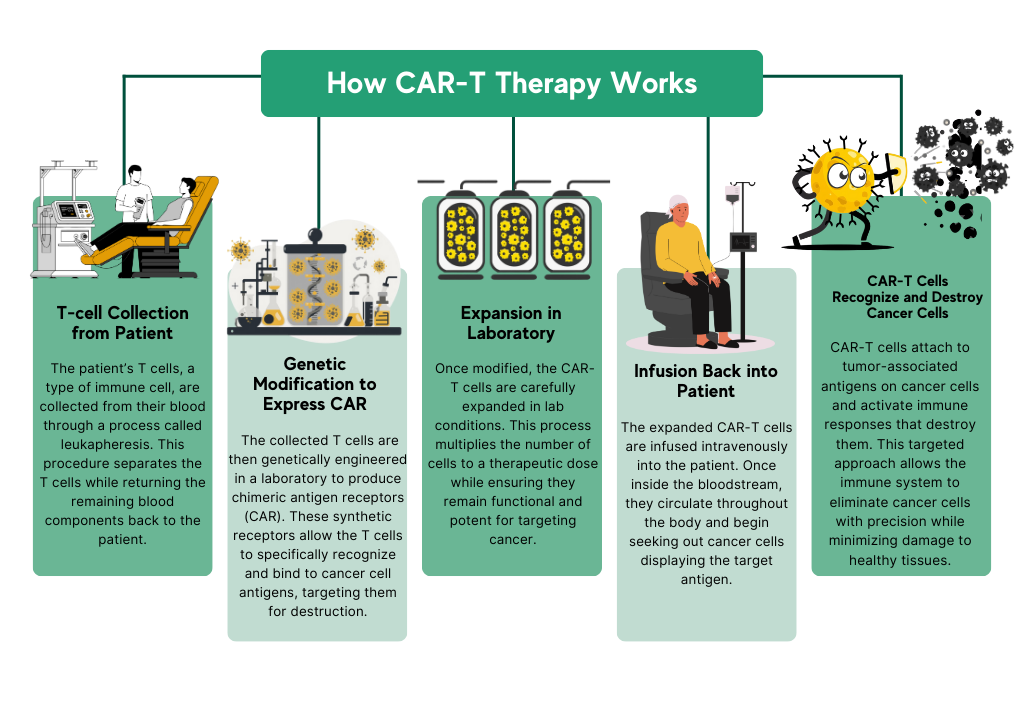
While initial breakthroughs have validated this approach in hematologic cancers, the next challenge lies in translating CAR-T therapy to solid tumors, where the tumor microenvironment, antigen heterogeneity, and immune evasion mechanisms present significant barriers to efficacy. This transition requires sophisticated preclinical models and translational strategies capable of accurately reproducing the complexity of human cancers.
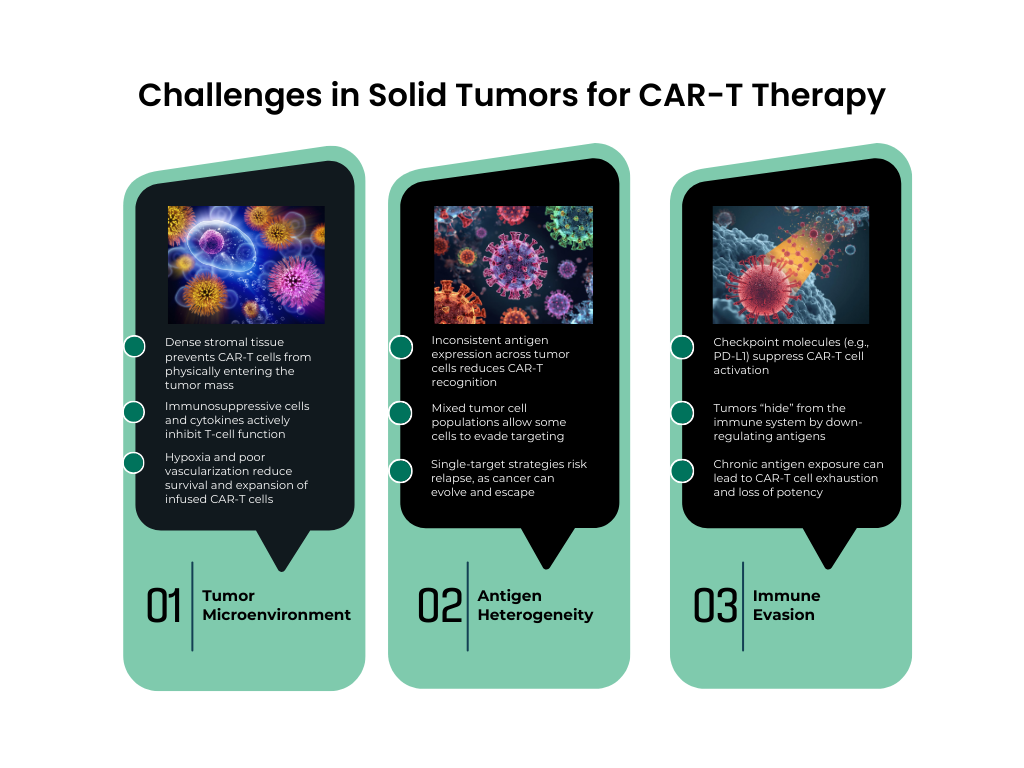
At Biotrial, we are deeply engaged in this next phase of CAR-T innovation. Our teams work with CAR-T cell therapies from the preclinical stage, using a comprehensive portfolio of solid tumor models, including humanized and syngeneic systems, to evaluate efficacy, safety, persistence, and tumor infiltration. Leveraging our expertise in immuno-oncology, pharmacology, and in vivo translational research, we generate high-quality data that inform mechanism-of-action studies and guide clinical program design.
Through close collaboration with our biotech and pharmaceutical partners, Biotrial aims to accelerate the translation of CAR-T therapies for solid tumors, helping to bridge the gap between experimental promise and clinical success.
At Biotrial, our multidisciplinary teams combine scientific rigor, technical expertise, and operational excellence at every stage of CAR-T therapy development. By integrating advanced preclinical models, robust translational research, and collaborative partnerships, we ensure that CAR-T candidates are rigorously evaluated and optimally positioned for clinical success. Our approach bridges the gap between experimental promise and clinical application, providing a comprehensive platform that supports innovation while maintaining focus on patient outcomes.
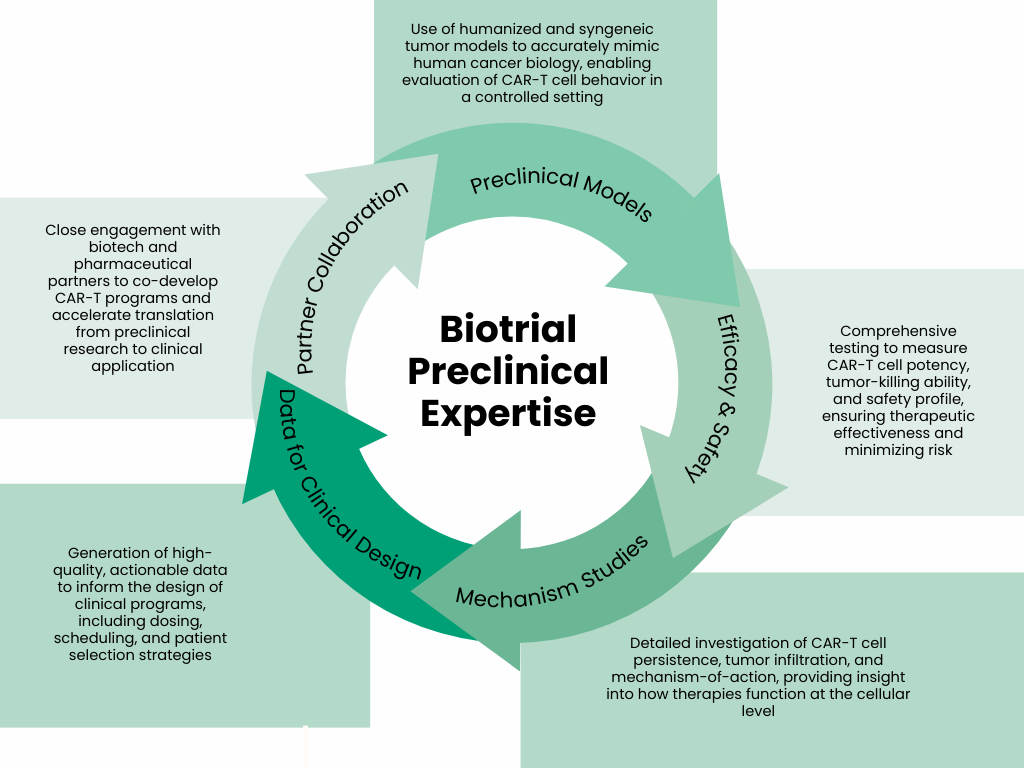
In summary, CAR-T therapy continues to redefine cancer treatment. With Biotrial’s expertise in preclinical research, advanced tumor models, and strong collaboration with biotech and pharmaceutical partners, we help transform promising CAR-T therapies into viable clinical candidates. Our methodical and data-driven approach ensures that each therapy is carefully assessed, strategically guided, and positioned to achieve meaningful impact in patients, bridging the critical gap between laboratory discovery and life-changing clinical outcomes.

