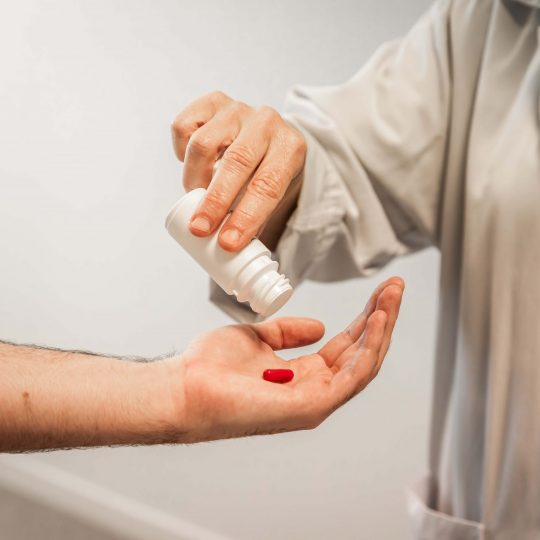
FIH (First-in-Human), SAD/MAD Trials
As a global CRO with over 35 years of experience in conducting FIH studies, Biotrial has a team of experienced early-phase study experts, who are well-versed in the design, conduct, and interpretation factors.
We have conducted FIH studies for a wide range of drugs, including small molecules, biologics, and gene therapies as well as in a variety of therapeutic areas, including oncology, cardiovascular disease, and neurodegenerative disorders.
Our phase 1 units in the US and France are located close to nearby hospital sites, which allows us to quickly and easily access medical care, equipment, and KOLs.